- Undergraduate
Bachelor's Degrees
Bachelor of ArtsBachelor of EngineeringPartner School Dual-DegreeUndergraduate AdmissionsUndergraduate Experience
- Graduate
Doctoral Degrees
Doctor of PhilosophyPhD Innovation ProgramDoctor of Medicine-PhDGraduate AdmissionsGraduate Experience
- Research
- Entrepreneurship
- Community
- About
-
All Thayer News
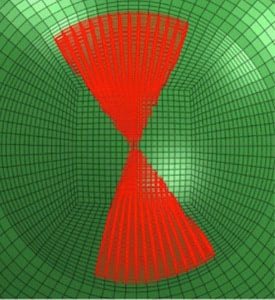
Linear bow tie zonal cross-linking pattern applied to flat axis. (Part of Avedro's theoretical model of photorefractive intrastromal cross-linking.)
China Food and Drug Administration (CFDA) Grants Approval for Avedro's KXL® System
Nov 11, 2015 | Avedro
Avedro is the first and only company to receive CFDA approval for corneal cross-linking
Avedro, Inc., an ophthalmic pharmaceutical and medical device company [founded by Dartmouth engineering professor Stuart Trembly], announces that it has received approval for its KXL Cross-Linking System from the China Food and Drug Administration (CFDA). The KXL Cross-Linking System is the first and only corneal cross-linking device to receive marketing approval in the People’s Republic of China. Approval of the KXL System is the culmination of a multi-year effort conducted by Avedro and its China distributor, Shinesee (Fujian) Ophthalmic Technology Development Co., Ltd.

Linear bow tie zonal cross-linking pattern applied to flat axis. (Part of Avedro's theoretical model of photorefractive intrastromal cross-linking.)
The cross-linking procedure combines riboflavin ophthalmic solution with UVA irradiation from Avedro’s KXL device to achieve corneal cross-linking and is indicated to treat keratoconus and post-LASIK ectasia, both of which are progressive, sight threatening conditions. The device was approved following an approval recommendation by the CFDA Special Expert Panel as well as mechanical and electrical testing by a CFDA certified national testing center and a safety and effectiveness review conducted by the Center for Medical Device Evaluation (CMDE).
Zhou Xing Tao, MD, PhD, professor of ophthalmology and director of the refractive surgery center, department of ophthalmology, Eye & ENT Hospital of Fudan University, Shanghai, China commented, “As the first ophthalmic surgeon in China to use the KXL System for corneal cross-linking, I have been clinically evaluating its safety and effectiveness for almost three years and found all related corneal parameters to be made more stable by cross-linking. I am very pleased with the approval of the KXL System because it now allows all patients in China who suffer from keratoconus and ectasia full access to corneal cross-linking.”
David Muller, PhD, CEO of Avedro states, “Securing approval in China is a significant milestone accomplishment for the Avedro team, our ophthalmic clinical investigators in China and our China distribution partner. China is the largest potential market in the world for corneal cross-linking with an estimated 1.4 million keratoconic eyes and more than 1000 refractive centers. This represents a virtually untapped opportunity for Avedro.”
Link to source:
For contacts and other media information visit our Media Resources page.
